Body Fluid Distribution
The Total fluid present in the human body is about 60 % of the total mass of the body, that is if any person weights 60 kg then the total amount of fluid present in their body is about 36kg.
Fluid present in our body is divided based on different compartment. For example, fluid which is present inside our cells including RBC (Red Blood Cell) are the intracellular fluid (ICF) which contributes around 2/3 of the total body fluid that is for 60kg individual, 36 multiple by 2/3 which is 24 kg of fluid at the ICF.
Whereas Extracellular Fluid (ECF) which contributes about 1/3rd of the Total body fluid that is for 60kg individual, it’s 12kg of fluid.
Extracellular fluid (ECF) consists of all body fluid present outside the cells, which consists of plasma fluid, interstitial fluid and some cations and anions.
Interstitial fluid contains 2/3 of the extracellular fluid whereas Plasma volume contains the remaining 1/3 of the extracellular fluid.
The Extracellular fluid (ECF) are separated from the Intracellular fluid (ICF) through a cell membrane which acts like a barrier for the Extracellular fluid and a relative barrier for Sodium (Na+) because it can't able to cross the cell membrane passively. As Cell membranes are semi-permeable in nature which allows fluid to pass across the membrane by Osmosis.
Surrounding the cells are the Interstitial fluid or tissue fluid which serve as a medium to provide the exchange of gases & nutrients to the cells.
Plasma volume is separated from interstitial fluid through a capillary and mostly contains water and contains dissolved proteins like fibrinogens, globulins, and albumins. Here Capillary membrane acts as a barrier to plasma proteins because plasma proteins are large particles which couldn't able to cross the capillary membrane.
Plasma volume also contains glucose, clotting factors, hormones and cations & anions. These dissolved substances are involved in many varied physiological processes in body, like gas exchange, immune system function, and for the distribution drug throughout the body.
The major cations which is present in our Intracellular Fluid are Potassium (K+) and Magnesium (Mg+²) whereas Phosphate (PO4-³) and Proteins are the major anions. As DNA, RNA & ATP which is present inside the cell contains Phosphate ion, whereas Proteins are produced by cells through translation are abundant anion in our cells and Intracellular compartment.
The major cations present in Extracellular fluid includes Sodium (Na+) and calcium (Ca2+) whereas major Anion includes are Chloride (Cl-) and hydrogen carbonate (HCO3-). As we take Salt in diet which is basically Na+ & Cl- ions that is what abundant in Extracellular compartment.
In order to measure the compartment of body fluid we use a tracer, a tracer is the substance which is specific to a particular compartment.
Albumin which is the most abundant protein present in the plasma fluid are used as a tracer to measure plasma compartment. Albumin which posses a large size couldn't able to cross the capillary membrane from plasma compartment.
To measure the Extracellular Compartment (ECF); Inulin, Mannitol, Sodium & Sucrose are used because they are limited to only Extracellular Compartment.
Likewise to measure the Total Body Water (TBW), we need a substance which can cross or permeable to both Cell membrane and Capillary membrane that is fulfilled by Tritiated water or Urea.
Here in this question, a data of body compartment is given in a 24 years old man. Let's discuss one by one.
Body weight is given 100kg which means that 60L is the total body water in this person as 60% of our body weight is water.
Here the Inulin space is given which is basically a Extracellular Compartment volume because Inulin is a tracer used to measure the Extracellular compartment (ECF). So, the Extracellular fluid is 20L out of 60L of Total body water (TBW). Hence, Intracellular Fluid in this person is (60-20)L that is 40L.
But Question here asked is to measure the Interstitial fluid volume which is (ECF - PLASMA VOLUME).
Plasma volume is given 4L means the Interstitial fluid volume would be (20 - 4)L that is 16L.
Hence Option B is correct.
Osmosis is the diffusion of water across the semi-permeable membrane where the water moves from the region of Hypotonic medium to Hypertonic medium when solutions are separated by a semi-permeable membrane.
As both Cell Membrane & Capillary Membrane are semi-permeable in nature. Cell Membrane separates Intracellular Fluid from Extracellular fluid whereas Capillary Membrane separates Interstitial fluid from Plasma volume which allows water to move from a region of Hypotonic medium to Hypertonic medium.
The pressure which created as a result of medium difference is an osmotic gradient which propel water to move across the membranes.
Let’s apply the concept of Osmosis to understand the pathology of diabetes Mellitus.
In patient suffering from Diabetes Mellitus, there is a deficiency of insulin either due decreased production of insulin or due to decrease activity of Insulin receptor.
Therefore, decrease in insulin unable the glucose to enter into the cell as insulin regulates the entry of insulin via GLUT 4 transporter.
As a result glucose started to build outside the cell which makes that medium Hypertonic compared to the cell, that's when the principle of osmosis started to play and water started to rush outside the cell into a Hypertonic medium.
The movement of water outside the cell brings the sign and symptoms of Diabetes Mellitus as cells started to shrink and excess fluid in the extracellular fluid started to come out as excessive urination (polyuria).
The hypotonicity and shrinking of the cells during Diabetes Mellitus causes an excessive thirst (polydypsia) and excessive hunger (polyphagia) in patient suffering from Diabetes Mellitus.
This picture demonstrates that how in the absence of insulin, Glucose concentration starts to backing up into the Extracellular compartment.
An increased glucose concentration at extracellular compartment makes the Glucose an effective osmole for Extracellular compartment as it can’t able to cross into the cell membrane due to either decreased production of insulin or due to decrease activity of Insulin receptor.
Likewise normally in our body Plasma Proteins and Sodium are the effective osmole for Vascular & Extracellular compartment.
Let's discuss some questions to solidify the concept on osmosis.
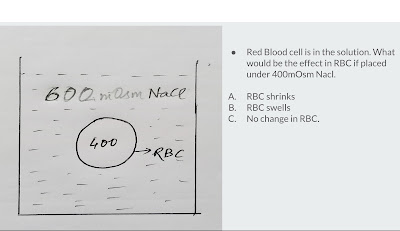
Red Blood cell (RBC) of 400mOsm osmolarity is placed in a container full of salt water(Nacl) solution.
If Red Blood Cell of 400mOsm is placed in a salt water(Nacl) solution of 200mOsm, then the solution osmolarity becomes less as compared to the Cell's osmolarity and Sodium (Na+) is an effective osmole of extracellular fluid. This means that there is net osmotic gradient force towards Red Blood Cells as fluid moves from Hypotonic medium to Hypertonic medium when medium are separated by semi-permeable membrane. Hence, Red Blood Cell swells.
If Red Blood Cell of 400mOsm is placed in a salt water(Nacl) solution of 600mOsm, then the solution osmolarity becomes more as compared to the Cell's osmolarity and Sodium (Na+) is an effective osmole of extracellular fluid. This means that there is net osmotic gradient force towards Nacl solution as fluid moves from Hypotonic medium to Hypertonic medium when the medium are separated by semi-permeable membrane. Hence, Red Blood Cell shrinks.
If Red Blood Cell of 400mOsm is placed in a Urea solution of 400mOsm, then the solution osmolarity becomes equal to the Cell's osmolarity but Urea is not the effective osmole of extracellular compartment that is it can cross cell membrane. This means that osmosis concept would fail here and movement of fluid is determined by the volume & pressure of the solution rather than concentration of the solution. Hence, Red Blood Cell starts to swell as volume of Urea is more as compared to volume of Red Blood cells.
The figure above represents a Basic metabolic panel (BMP) which is also called Chem-7 used in basic blood profile test.
The lab values indicate that Sodium (Na+) is the most abundant osmole of the extracellular space which is also an effective osmole of extracellular space.
Lab values of Blood Urea Nitrogen (BUN), Creatinine & Glucose are measured in Mg/dl or mg% while others are measured in mEq/L or Mmol/L.
Under osmotic equilibrium, when the fluid compartments are in equilibrium and there is no movement of fluid between Intracellular & Extracellular compartment, the osmolarity of both the compartments becomes equal in order to maintain the equilibrium.
Therefore, We can measure the total milliosmoles under osmotic equilibrium with the help of Extracellular fluid osmolarity and Total Body water. As under osmotic equilibrium both intracellular & extracellular compartment have equal osmolarity which is measured in mOsm/L. So by multiplying the mOsm/L with Total Body water in Liters (L) then liters would cut out and we get the total milliosmoles of the given solution.
Osmolar gap is the difference between estimated and measured osmolarity where Estimated osmolarity is the calculated osmolarity based on patient data whereas Measured osmolarity is the measured osmolarity from an instrument osmometer.
Normally, 290 mOsm/l is the normal osmolarity found in a normal blood profile data.
However if the difference between measured and estimated osmolarity exceeds 15 then it indicates the presence of ethanol, methanol, ethylene glycol, acetone or mannitol in the body.
Let's do some questions to solidify the concept on Osmolar gap and osmolarity.
In this question, a patient is bought to the emergency room with a fractured occiput and unresponsive. We took their Blood profile data upon admission and his measured osmolarity is 356 mOsm/kg.
We then approach to calculate whether he has a Osmolar gap.
ECF Estimated Osmolarity = 2(Na)+ glucose/18 + BUN/2.8
So, 2×(Na) + glucose/18 + BUN/2.8
= 2 × 143 + 104 /18 + 4/2.8
= 286 + 5.7 + 1.4
= 293.1 ≈ 293 mOsm/kg
Therefore,
Osmolar gap=Measured Osm.– Estimated Osm.
= 356 mOsm/kg – 293 mOsm/kg
= 63 mOsm/kg
Hence, There is a significant Osmolar gap(>15) which indicates the presence of Ethanol, Methanol, Ethylene glycol, Acetone or Mannitol in the body.
In this question, a person consumes 3 L of water and 10 mEq of potato chips and we need to estimate his new extracellular osmolarity assuming osmotic equilibrium.
So, as we discussed that under osmotic equilibrium, extracellular and intracellular osmolarity becomes same.
Therefore,
Total milliosmoles = Extracellular fluid Osmolarity × Total Body Water (TBW)
Extracellular fluid Osmolarity = 285mOsm/kg (given)
Total Body water = ICF + ECF
= 14 L + 28 L
= 42 L
= 42 kg
Total milliosmoles = 285 mOsm/kg × 42kg
= 11970 mOsm
After Consuming 3 L of water and 10 mEq of sodium.
New milliosmoles = (11970 + 10) mOsm
= 11980 mOsm
New Total Body Water = (42 + 3) L
= 45 L
Hence,
New Extracellular fluid Osmolarity = New milliosmole /New Total Body Water
New ECF = (11980/45) mOsm/kg
= 266 mOsm/kg
























Leave a Comment